The Belgian competent authority (AFMPS/FAGG) has updated the form for the Free Sales Certificates (FSC) under the Medical Devices Regulation 2017/745 (MDR) and the In Vitro Diagnostic Devices Regulation 2017/746 (IVDR).


The Belgian competent authority (AFMPS/FAGG) has updated the form for the Free Sales Certificates (FSC) under the Medical Devices Regulation 2017/745 (MDR) and the In Vitro Diagnostic Devices Regulation 2017/746 (IVDR).

The coming into effect of the MDR has meant several new changes for manufacturers. Among those, the MDR has set a new classification category for Class I devices: the reusable surgical instruments (Class Ir).

The coming into effect of the MDR has meant several new changes for manufacturers. Among those, the MDR has set a new classification category for Class I devices: the reusable surgical instruments (Class Ir).

The coming into effect of the MDR has meant several new changes for manufacturers. Among those, the MDR has set a new classification category for Class I devices: the reusable surgical instruments (Class Ir).

The coming into effect of the MDR has meant several new changes for manufacturers. Among those, the MDR has set a new classification category for Class I devices: the reusable surgical instruments (Class Ir).
Non-EU manufacturers can sell medical devices on the European Union market only if they appoint an authorised representative (EAR). Read more!

Article 10 (16) of the MDR obliges the manufacturer to have sufficient financial coverage for potential liability of defective medical devices. Read more!

Articles 31 of the MDR and Article 28 of the IVDR require all manufacturers, authorised representatives, and importers to register as actors on the EUDAMED database.
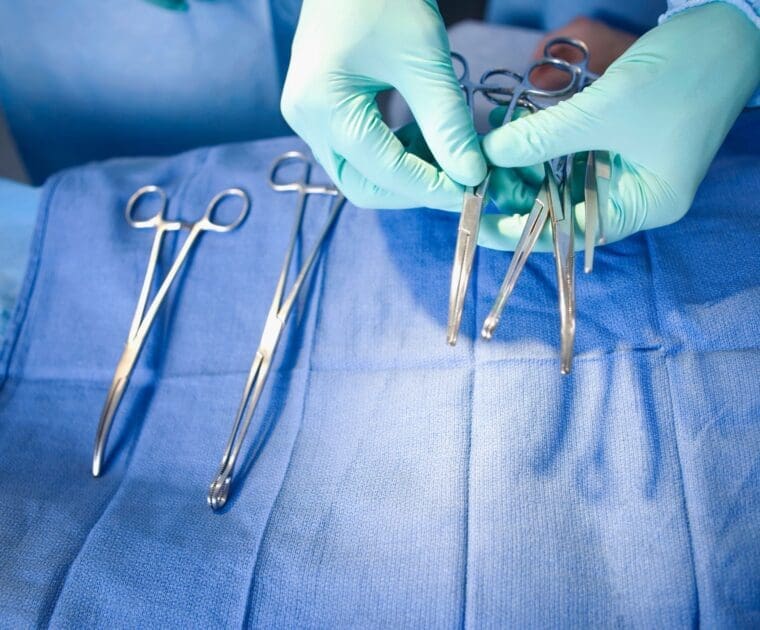
The coming into effect of the MDR has meant several new changes for manufacturers. Among those, the MDR has set a new classification category for Class I devices: the reusable surgical instruments (Class Ir).
The EU IVDR (Regulation on in-vitro diagnostic devices) applies as of May 26, 2022, profoundly…